- 17 December
-

Medsintez Plant, one of the leading pharmaceutical companies in the Urals, partnered with the 15th Anniversary Festival of Expectant Mothers in the Sverdlovsk Region. The event, aimed at strengthening family values and supporting motherhood, was held in Ekaterinburg with the support of the regional government and as part of the national Family Project.
- 27 November
-

The entry of the Russian antiviral drug TRIAZAVIRIN® into the South African pharmaceutical market opens new opportunities not only for international healthcare cooperation but also for enhancing the health of patients in both countries.
- 05 November
-
Medsintez Plant participated in a key industry event, Product Innovation Fair in Medicine and Healthcare, which took place in Moscow on October 29–30. Viktor Fisenko, First Deputy Minister of Healthcare of Russia, attended the exhibition of domestic innovations in the field of drugs and medical products.
- 25 October
-

The 3d Scientific and Practical Conference The Ural Peaks. Respiratory Infections 2025 was held in Ekaterinburg on October 15–16 as part of the 4th International Forum Days of Virology 2025. The event organized by the Ministry of Health of the Sverdlovsk Region, the Smorodintsev Research Institute of Influenza, and the Ural State Medical University brought together over 1,100 participants in person and online from 36 cities across Russia.
- Home
- Cluster members
- Nipro PharmaPackaging Ural LLC
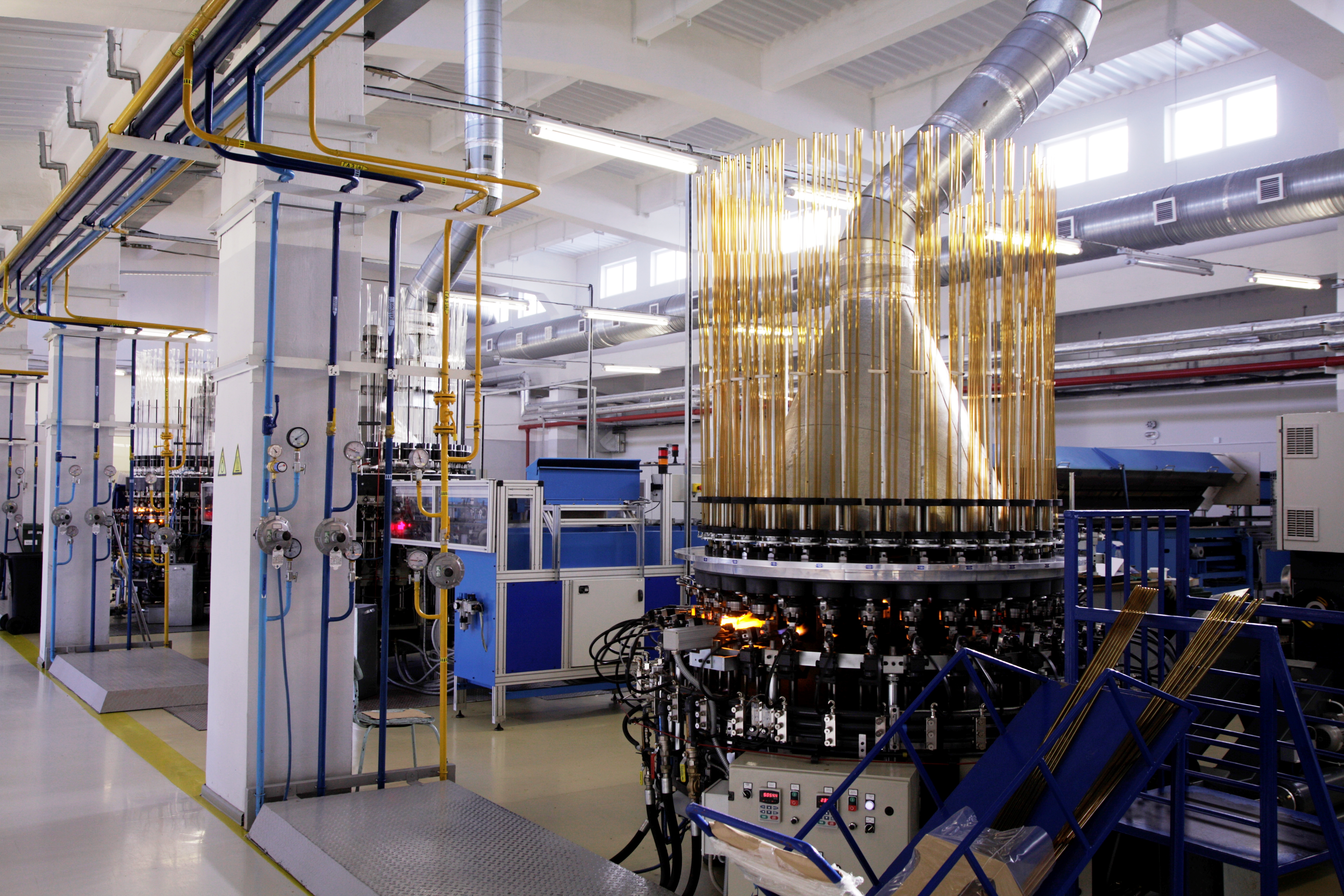
Nipro PharmaPackaging Ural LLC

Nipro PharmaPackaging Ural LLC is a 100% subsidiary of NIPRO (Japan).
The company is engaged in manufacture of primary glass packaging for pharmaceutical industry.
Ampoules, vials, cartridges are made exclusively of a glass tube of the 1st hydrolytic class Type I, in accordance with European, American, Japanese pharmacopoeias, of the following brands: NSV51 (manufacturer – Nipro PharmaPackaging), Fiolax (manufacturer Schott), FS5.0 (manufacturer Four Stars, China), JUNHENG (manufacturer – Triumph Junheng, China), Corning-51-D (China).
The range of products manufactured by the company includes:
• Ampoules: 1 to 25 ml, forms B, C, D, E; made of clear and amber glass, with open point cut or cut ring, in accordance with ISO 9187 It is possible to apply marker rings to the ampoules.
• Vials: 1 to 30 ml, made of clear and amber glass, with a crimp neck, in accordance with ISO 8362
• Cartridges with a nominal capacity of 1.5; 1.8; 3 ml; made of clear glass in accordance with ISO 13926
Technological lines are equipped with options for printing on products, which allows you to apply a company logo or other information, for additional protection of medicines against counterfeiting.
In addition to standard products, the company is capable to manufacture products in accordance with individual customer drawings.
otal production capacity is up to 300 million items per year. The plant is equipped with the latest equipment: European made ampoule and vial lines by OCMI (Italy), Euromatic (Italy), NCE (Belgium), a cartridge lines of own production by NIPRO (France). All technological lines are equipped with automated quality control systems VIMEC (Netherlands), which allows rejecting products for geometric, cosmetic and mechanical defects.
The company implemented a quality management system certified in accordance with ISO 9001:2015, ISO 15378 (Special requirements for ISO 9001:2008 implementation, taking into account Good Manufacturing Practice GMP).
Ilya Markovsky,
Director
+7 (912) 242-74-99