- 17 December
-

Medsintez Plant, one of the leading pharmaceutical companies in the Urals, partnered with the 15th Anniversary Festival of Expectant Mothers in the Sverdlovsk Region. The event, aimed at strengthening family values and supporting motherhood, was held in Ekaterinburg with the support of the regional government and as part of the national Family Project.
- 27 November
-

The entry of the Russian antiviral drug TRIAZAVIRIN® into the South African pharmaceutical market opens new opportunities not only for international healthcare cooperation but also for enhancing the health of patients in both countries.
- 05 November
-
Medsintez Plant participated in a key industry event, Product Innovation Fair in Medicine and Healthcare, which took place in Moscow on October 29–30. Viktor Fisenko, First Deputy Minister of Healthcare of Russia, attended the exhibition of domestic innovations in the field of drugs and medical products.
- 25 October
-

The 3d Scientific and Practical Conference The Ural Peaks. Respiratory Infections 2025 was held in Ekaterinburg on October 15–16 as part of the 4th International Forum Days of Virology 2025. The event organized by the Ministry of Health of the Sverdlovsk Region, the Smorodintsev Research Institute of Influenza, and the Ural State Medical University brought together over 1,100 participants in person and online from 36 cities across Russia.
- Home
- Cluster members
- MedicorPharma-Ural LLC
MedicorPharma-Ural LLC

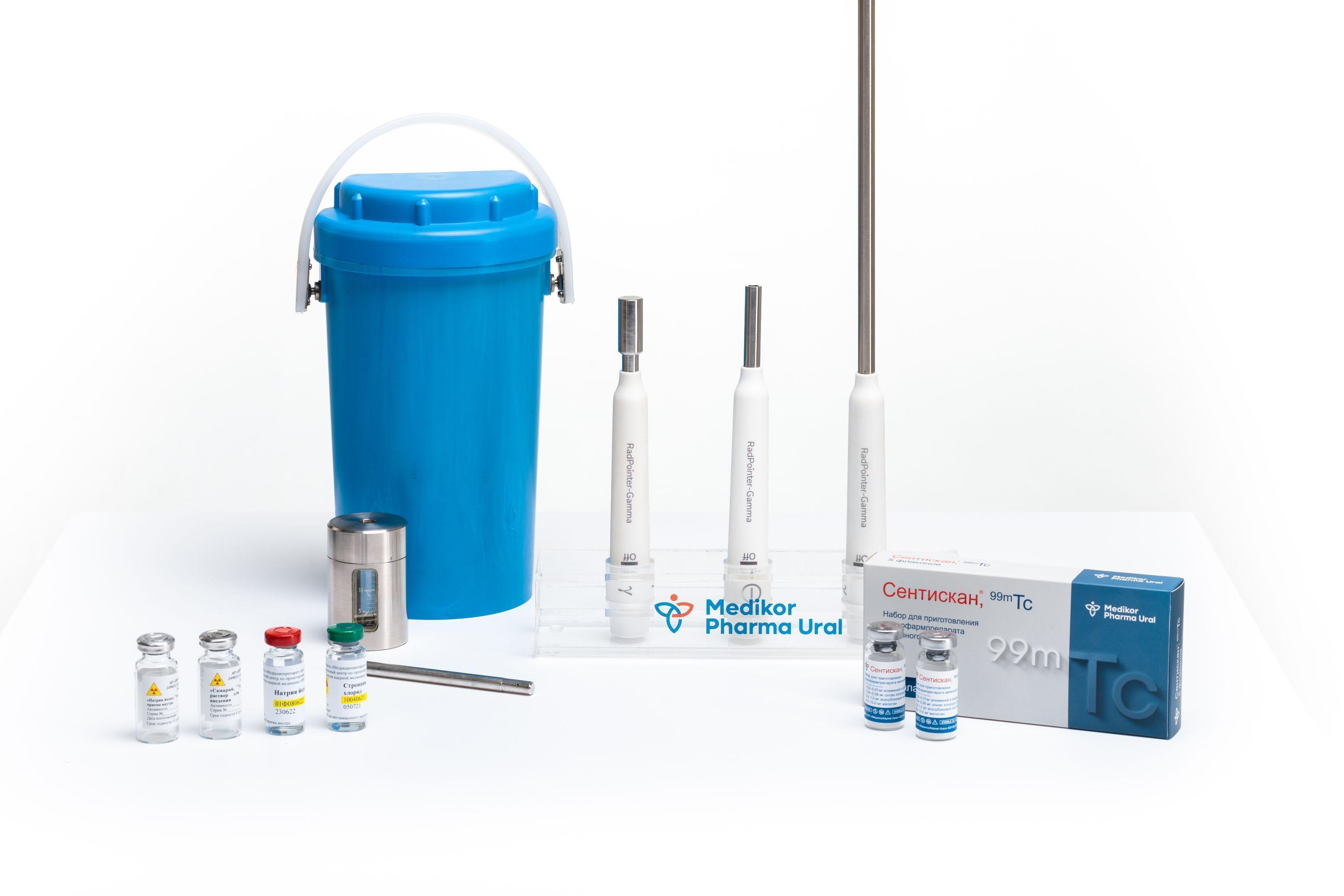
MedicorPharma-Ural LLC has been a supplier of equipment for nuclear medicine and radioisotope products to healthcare institutions throughout Russia and abroad for more than 10 years.
Products supplied:
- radiopharmaceuticals based on I-131, Sm-153, Sr-89;
— technetium Тс-99m generators;
— microsources of radioactive emission based on I-125;
— intraoperative gamma-probe RadPointer;
— kits for the preparation of radiopharmaceuticals labeled with 99mTc;
— other products for nuclear medicine.
MedicorPharma-Ural LLC provides services for:
— support in customs clearance of radioisotope medical products for export and import operations;
— transportation of radioactive cargo (hazard class 7);
— development of projects for bringing goods and services to the Russian market;
— registration of the shipping packaging set;
— training in the use of medical devices and radiopharmaceuticals; – and others.
The company is a manufacturer of the RadPointer gamma- probe and 99mTc-labeled radiopharmaceutical preparation kits for high-tech patient care.

The RadPointer Intraoperative Gamma-Probe is designed to search for and localize sentinel lymph nodes during diagnostic and surgical procedures.
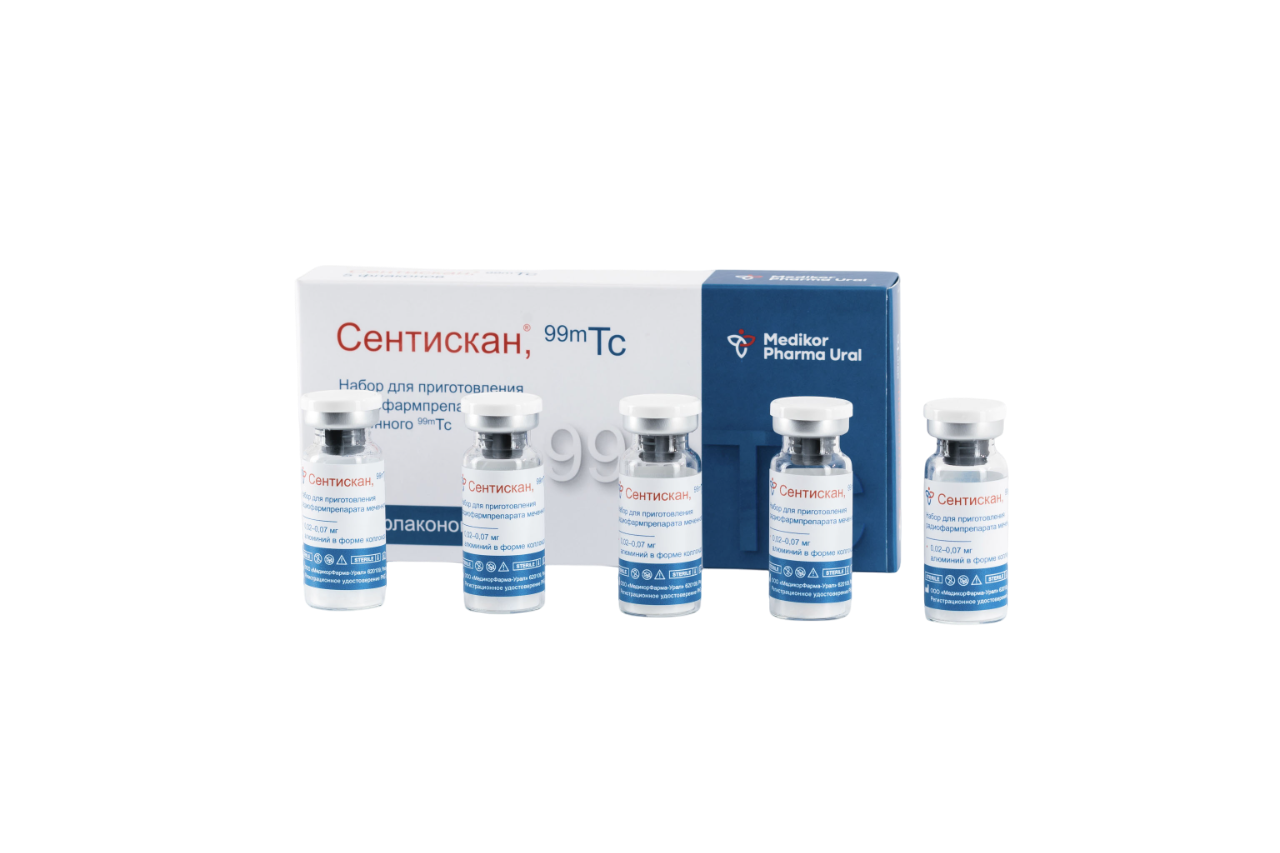
Sentiscan®, 99mTc is a kit for the preparation of a radiopharmaceutical labeled with 99mTc, which is a nanocolloidal aluminum solution.
It is used for scintigraphic imaging and intraoperative detection of sentinel lymph nodes in patients with various malignant neoplasms (including breast, melanoma of the skin, larynx and laryngopharynx, oral mucosa, vulva, cervix, endometrium, etc.).
Also, the line of kits for the preparation of diagnostic radiopharmaceuticals produced by MedicorPharma-Ural LLC includes drugs for the diagnosis of malignant tumors of the prostate, brain and neuroendocrine tumors.
Disinfectant (skin antiseptic) DEZITOL A
Skin antiseptic based on isopropyl alcohol for hand treatment, including hands of surgeons, operating field, injection field, gloves on the hands of medical personnel (in accordance with the instructions for use). Having high antimicrobial activity, it is gentle for skin. Effective against pathogens of healthcare-associated infections and Mycobacterium tuberculosis (tested on Mycobacterium terrae).
Passed tests at Disinfectology Research Institute of the Federal Service for the Oversight of Consumer Protection and Welfare.
Effective against pathogens of healthcare-associated infections and Mycobacterium tuberculosis (tested on Mycobacterium terrae).
It is intended for hygienic treatment of hands of personnel at industrial enterprises, catering facilities, municipal facilities, sanitary inspection checkpoints, hairdressing salons, beauty salons, etc.

MedicorPharma-Ural LLC has all the necessary licenses, including a license to operate radiation sources and to carry out pharmaceutical activities. It is also certified according to international operating standards ISO 9001:2015 (GOST R ISO 9001-2015 Quality management systems. Requirements), ISO 13485:2017 (GOST ISO 13485-2017 Medical devices. Quality management systems. Requirements for regulatory purposes).
28/1, Kirova St., Ekaterinburg
+7 (343) 270-75-29
8 800 600 33 90


































































































































